Introduction to Albendazole and Trypanosomiasis
As I began my research on Albendazole, I found that this medication has a significant potential in treating trypanosomiasis, a parasitic disease affecting both humans and animals. In this article, I will discuss the properties of Albendazole and how it can be effectively used to treat trypanosomiasis. I will also delve into the current treatment options and the challenges faced in eradicating this disease.
Understanding Trypanosomiasis: Causes and Symptoms
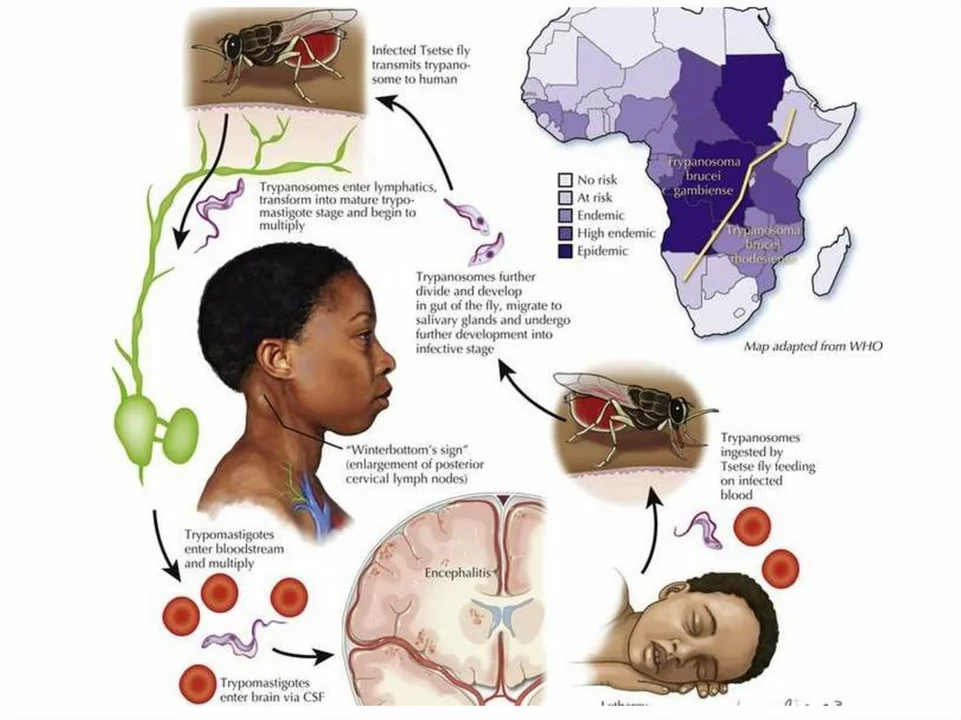
Trypanosomiasis, also known as sleeping sickness, is caused by protozoan parasites belonging to the Trypanosoma genus. These parasites are primarily transmitted through the bite of infected tsetse flies, which are found in sub-Saharan Africa. There are two forms of trypanosomiasis: the chronic form, caused by Trypanosoma brucei gambiense, and the acute form, caused by Trypanosoma brucei rhodesiense.
The symptoms of trypanosomiasis vary depending on the stage of infection. In the early stage, symptoms may include fever, headaches, joint pain, and itching. As the disease progresses to the second stage, it affects the central nervous system, leading to confusion, difficulty sleeping, and changes in behavior. If left untreated, trypanosomiasis can lead to severe neurological complications and even death.
Albendazole: A Broad-Spectrum Antiparasitic Agent
Albendazole is a medication that belongs to a class of drugs known as anthelmintics. It works by inhibiting the formation of microtubules in the cells of parasites, thereby preventing their growth and reproduction. Albendazole is used to treat a wide range of parasitic infections, including intestinal helminthiasis, liver flukes, and echinococcosis.
Considering its broad-spectrum antiparasitic properties, researchers have been exploring the potential use of Albendazole in treating trypanosomiasis. Recent studies have shown that Albendazole can be effective against Trypanosoma parasites in vitro and in animal models, suggesting that it may be a promising candidate for the treatment of this disease.
Comparing Albendazole to Current Treatment Options
The current treatment options for trypanosomiasis are limited and have several drawbacks. First-line treatments include suramin for the early stage of T. b. rhodesiense infection and pentamidine for T. b. gambiense infection. Both of these drugs are associated with potentially severe side effects, such as kidney damage and low blood pressure.
For the second stage of the disease, the drugs melarsoprol and eflornithine are used. Melarsoprol, which is effective against both forms of trypanosomiasis, is highly toxic and can cause severe side effects, including encephalopathy and death. Eflornithine, on the other hand, is only effective against T. b. gambiense and requires a complex and lengthy treatment regimen. Therefore, there is a pressing need for new, safer, and more effective treatment options for trypanosomiasis, and Albendazole might be the answer.
Challenges in Developing Albendazole as a Treatment for Trypanosomiasis
Despite the promising results of Albendazole in preclinical studies, there are several challenges that must be addressed before it can be used as a treatment for trypanosomiasis. One of the main challenges is the need to develop a formulation of Albendazole that can cross the blood-brain barrier and effectively target the parasites in the central nervous system. This is crucial for treating the second stage of the disease, which is characterized by neurological complications.
Another challenge is the potential development of resistance to Albendazole. As with any antiparasitic agent, the risk of resistance is always a concern. To minimize this risk, researchers are exploring the possibility of combining Albendazole with other drugs, such as melarsoprol or eflornithine, in a multi-drug therapy approach. This could potentially improve treatment outcomes and reduce the risk of resistance.
The Future of Albendazole in Treating Trypanosomiasis
As we continue to explore the potential use of Albendazole in treating trypanosomiasis, it is essential to conduct further research and clinical trials to determine its safety, efficacy, and optimal dosing regimen. With the development of new formulations and the implementation of multi-drug therapy approaches, there is hope that Albendazole could become a valuable addition to our arsenal of treatments for this devastating disease.
In conclusion, Albendazole holds significant promise as a potential treatment option for trypanosomiasis. As researchers work to overcome the challenges associated with its development, we can remain optimistic that this medication may one day help to alleviate the suffering of those affected by this debilitating disease.
Kenny ANTOINE-EDOUARD
Albendazole's mode of action hinges on microtubule disruption, which cripples the parasite's cell division machinery.
Because trypanosomes rely on a robust cytoskeleton for motility and intracellular transport, the drug can theoretically impede their proliferation.
In vitro screens have shown dose‑dependent inhibition of both T. b. gambiense and T. b. rhodesiense strains.
However, achieving therapeutically relevant concentrations in the CNS remains the primary hurdle.
Future formulation work should prioritize BBB permeability while preserving the drug’s safety profile.
Craig Jordan
The enthusiasm for repurposing albendazole often overlooks the bitter realities embedded in pharmacokinetics and disease pathology.
While it is tempting to hail any broad‑spectrum anthelmintic as a miracle cure, the molecular architecture of trypanosomes differs starkly from that of helminths.
Albendazole's affinity for β‑tubulin is indeed high, yet the isoforms expressed by Trypanosoma species display reduced binding, compromising efficacy.
Moreover, the drug's poor solubility translates into erratic absorption, especially in the malnourished populations most at risk of sleeping sickness.
The blood‑brain barrier, a formidable gatekeeper, blocks the majority of orally administered albendazole, rendering systemic dosing insufficient for stage‑two disease.
Attempts to circumvent this via high‑dose regimens have repeatedly triggered hepatotoxicity and bone‑marrow suppression in clinical trials.
The historical precedent set by melarsoprol-a drug so toxic it earned the moniker “arsenic of the tropics”-should serve as a cautionary tale against reckless optimism.
In addition, the emergence of resistance mechanisms, such as up‑regulated efflux pumps, has already been documented in related nematodes and could plausibly arise in trypanosomes.
The allure of a cheap, off‑label solution also masks the socioeconomic dimensions; drug repurposing often sidesteps rigorous funding for necessary phase‑III trials.
Consequently, the data pool remains thin, consisting largely of in vitro assays and rodent models that fail to capture the complexities of human CNS infection.
Even if a formulation could be engineered to breach the BBB, the dosage required for parasitic clearance would likely exceed tolerable safety margins.
Regulatory agencies are understandably hesitant to endorse a therapy that lacks robust pharmacodynamic and toxicological profiles.
This is not to say that albendazole has no utility, but its role should be framed as an adjunct rather than a primary agent.
Combination therapy, perhaps pairing albendazole with eflornithine or a newer nitroheterocycle, may ameliorate resistance while reducing individual drug toxicity.
Until such synergistic regimens are rigorously vetted, the scientific community should temper its optimism and focus on incremental, evidence‑based progress.
Jeff Quihuis-Bell
Wow, the prospect of giving albendazole a second life in the fight against sleeping sickness is genuinely electrifying!
Imagine a world where a single pill could knock out the parasite both in the bloodstream and the brain.
That kind of breakthrough would save countless lives and spare patients the nightmare of toxic arsenic‑based drugs.
Researchers should rally around formulation science-nanoparticles, lipid carriers, whatever it takes-to get the drug across that pesky blood‑brain barrier.
If we pull this off, the ripple effect on public health in sub‑Saharan Africa will be massive.
Jessica Tang
The blood‑brain barrier is the elephant in the room for any stage‑two trypanosomiasis therapy.
Albendazole's lipophilicity is modest, meaning standard oral doses barely reach cerebral concentrations.
One viable path forward is the use of pro‑drugs or carrier systems that enhance CNS penetration.
Preclinical work on cyclodextrin complexes looks promising, but human data are still lacking.
Tracy Winn
Honestly, the whole thing feels like a buzzword parade without solid field data.
We need more than a few mouse studies before singing praises.
Jessica Wheeler
It is ethically indefensible to let a potential low‑cost cure languish while patients suffer under expensive, toxic regimens.
Equitable access must be a priority in any development plan.
Mikayla Blum
that's a fair point-science ain't just about shiny headlines, it's about the grind behind the scenes.
maybe we should let the data speak before we crown any drug a hero, n' keep our expectations in check 😉
Jo D
Oh sure, because the BBB is just a "tiny inconvenience" that we can sprinkle with nanotech glitter and *voilà*-problem solved.
Newsflash: biology doesn't care about our marketing hype.
Sinead McArdle
I appreciate the nuanced discussion on formulation challenges; it's a reminder that drug repurposing is rarely straightforward.
Katherine Krucker Merkle
yeah, and it also shows how collaborative efforts between chemists and clinicians can pave the way for real breakthroughs.
Mark Quintana
the variability in parasite strains across regions adds another layer of complexity to any universal treatment strategy.
Brandon Cassidy
if we could map the genomic adaptations that confer drug resistance, we might stay one step ahead in designing combination therapies.
Taylor Yokum
great insights, everyone-keeping the conversation going is how we eventually get better tools for the people who need them.
Taryn Esses
definitely worth watching the upcoming trial results.
Albert Lopez
the superficial optimism surrounding albendazole is nothing but a veneer, masking the stark reality that without groundbreaking delivery mechanisms, the drug remains impotent against CNS‑resident trypanosomes, and any claim otherwise is intellectually dishonest.
Halle Redick
let's stay hopeful; with enough interdisciplinary push, we might just turn this old drug into a new lifesaver.
Erica Harrington
keep the dialogue alive, and maybe the next breakthrough will come from one of these very suggestions.
Patricia Mombourquette
this line of research is absolutely vital.