When you hear "breast cancer," you might think of one disease. But it’s not. HER2-positive breast cancer is a distinct type - aggressive, fast-growing, and once one of the hardest to treat. Today, it’s one of the most treatable. How? Because of targeted therapies that lock onto the HER2 protein like a key in a lock. These drugs don’t just attack cancer blindly. They go straight for the source. And that’s changed everything.
What Exactly Is HER2-Positive Breast Cancer?
Every breast cancer cell has proteins on its surface. Some of these proteins act like antennas, picking up signals that tell the cell to grow. HER2 is one of them. In HER2-positive breast cancer, the cells make way too many HER2 proteins - five to ten times more than normal. This floods the cell with growth signals, making it multiply like crazy. About 15% to 20% of all breast cancers are HER2-positive. It’s not rare. And before targeted drugs, it was one of the deadliest subtypes.
Doctors test for HER2 using two main methods: IHC (immunohistochemistry) and FISH (fluorescence in situ hybridization). If the test shows strong HER2 overexpression, you’re HER2-positive. That diagnosis used to mean a grim outlook. Now? It means you have options.
The First Breakthrough: Trastuzumab and Its Biosimilars
Trastuzumab - better known by its brand name Herceptin - was the first drug to change the game. Approved in the late 1990s, it was a game-changer. It’s a monoclonal antibody, meaning it’s made in a lab to look like a natural immune system protein. It binds tightly to the HER2 protein, blocking the growth signals and marking the cancer cell for destruction by the immune system.
Today, trastuzumab isn’t just one drug. There are biosimilars - near-identical copies - like Kanjinti, Ogivri, Ontruzant, Herzuma, and Trazimera. These work the same way but cost less. They’re used in both early-stage and metastatic cancer. For early-stage, patients typically get trastuzumab for a full year after surgery and chemo. For advanced cancer, it’s often combined with other drugs.
But trastuzumab has limits. It doesn’t cross the blood-brain barrier well. That means if cancer spreads to the brain - which happens often in HER2-positive cases - trastuzumab alone won’t help much.
Doubling Down: Trastuzumab + Pertuzumab
Why stop at one blocker? Pertuzumab (Perjeta) targets a different part of the HER2 protein. While trastuzumab latches onto one area, pertuzumab stops HER2 from teaming up with other receptors. Together, they block growth signals from every angle. This combo, called dual HER2 blockade, became standard for larger tumors (>2 cm) after the KRISTINE trial showed it shrank tumors better than trastuzumab alone.
There’s even a single-shot version: Phesgo. It combines trastuzumab, pertuzumab, and hyaluronidase into one subcutaneous injection. Instead of sitting in a clinic for 90 minutes for an IV drip, you get a 5- to 8-minute injection. Patients say it’s life-changing. Less time away from work. Less stress. More control.
The Next Leap: Antibody-Drug Conjugates (ADCs)
ADCs are like smart missiles. They’re antibodies (like trastuzumab) attached to a powerful poison. The antibody finds the HER2 protein. Then, it delivers the chemo directly inside the cancer cell. This means more damage to the tumor - and less harm to the rest of your body.
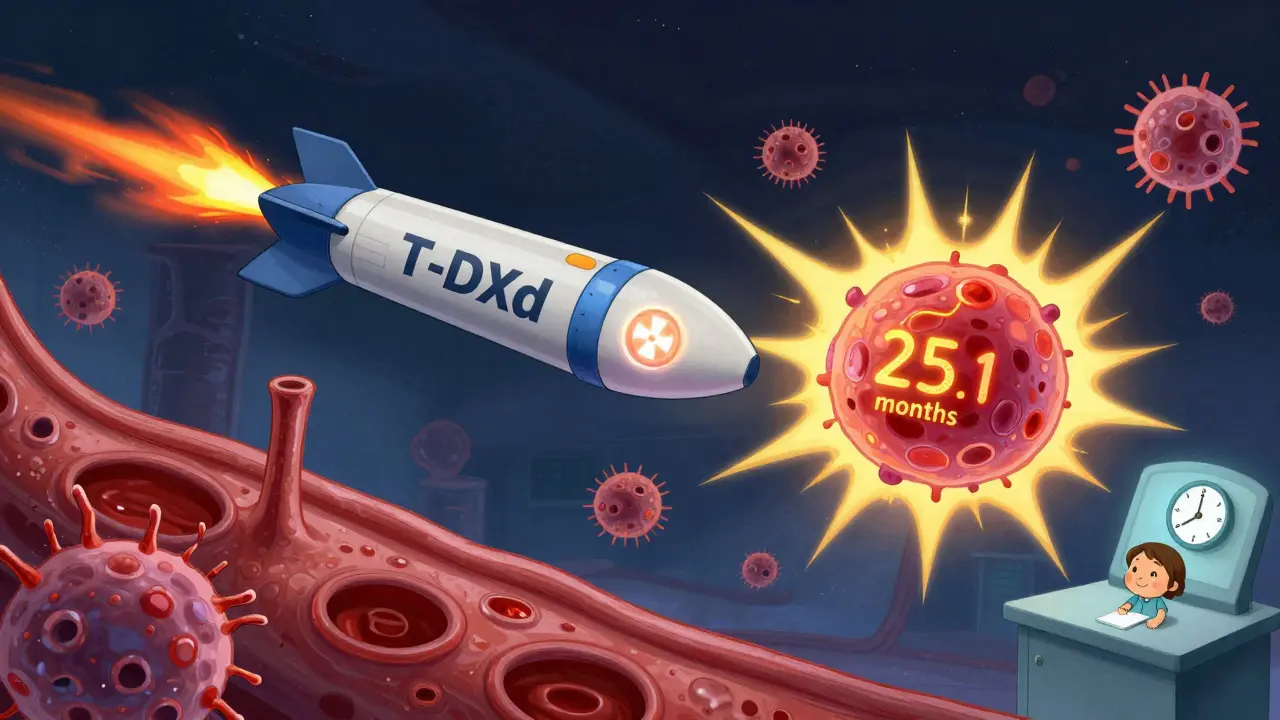
The first big ADC was T-DM1 (Kadcyla). It combined trastuzumab with a chemo called emtansine. It was a step up from trastuzumab alone. But then came T-DXd (Enhertu). This one uses a newer, more potent chemo payload and a clever linker that lets the drug leak out a little, killing nearby cancer cells too. That’s called the "bystander effect."
DESTINY-Breast03, a major 2021 trial, compared T-DXd to T-DM1 in patients who had already tried trastuzumab and pertuzumab. The results were stunning. T-DXd cut the risk of disease worsening or death by 72%. Median progression-free survival jumped from 6.8 months to 25.1 months. That’s more than triple the time before cancer got worse.
But T-DXd isn’t perfect. It carries a boxed warning for interstitial lung disease - a rare but serious lung inflammation. About 10-15% of patients get some form of it. Most are mild, but some need to stop treatment. Still, for many, the trade-off is worth it.

Tackling Brain Metastases: Tucatinib
Brain metastases are a nightmare in HER2-positive breast cancer. Traditional drugs like trastuzumab can’t reach them. That’s where tucatinib (Tukysa) comes in. It’s a small molecule tyrosine kinase inhibitor - a pill that slips right through the blood-brain barrier.
The HER2CLIMB trial tested tucatinib with trastuzumab and capecitabine in patients with brain mets. Results? A 46% reduction in risk of death. Median overall survival jumped from 17.4 months to 21.9 months. Progression-free survival went from 5.6 months to 7.8 months. For patients with brain tumors, this wasn’t just an improvement - it was a breakthrough.
Tucatinib also helps outside the brain. It’s now used in advanced cases even without brain involvement. The catch? It causes diarrhea. Severe diarrhea in about 10% of patients. Doctors often prescribe loperamide before the first dose and tell patients to keep it on hand.
Other Players: Neratinib, Lapatinib, Margetuximab
Neratinib (Nerlynx) is another TKI, used after trastuzumab in early-stage cancer to prevent recurrence. It’s taken daily for a year. But diarrhea? It’s brutal. Up to 40% of patients get grade 3 diarrhea. Prophylactic loperamide is mandatory.
Lapatinib (Tykerb) was one of the first TKIs. It’s rarely used now because newer drugs are better. But it still shows up in some cases.
Margetuximab (Margenza) is newer. It’s a monoclonal antibody designed to boost the immune system’s attack on HER2 cells. It’s approved for metastatic disease after at least two other HER2 therapies. It’s not a first-line drug - it’s a backup. But for patients who’ve tried everything else, it’s a lifeline.
Side Effects You Need to Know
Targeted therapies are better than chemo. No more vomiting. Less hair loss. But they have their own risks.
- Heart problems: Trastuzumab and pertuzumab can weaken the heart. About 2-7% of patients develop heart failure. That’s why doctors check your ejection fraction before and every 3 months during treatment.
- Lung issues: T-DXd can cause interstitial lung disease. A persistent cough? Tell your doctor. Don’t wait.
- Diarrhea: TKIs like neratinib and tucatinib cause it. Loperamide is your friend. Take it as prescribed.
- Low platelets: T-DM1 can drop your platelet count. You might bruise easily or bleed longer. Monitor it.
These side effects aren’t deal-breakers - they’re manageable. But you need to know them. And you need to speak up.
What’s Next? The Future of HER2 Therapy
The field is moving fast. HER2-low - once considered "not HER2-positive" - is now a category. If your cancer tests IHC 1+ or 2+ with negative FISH, you’re HER2-low. And T-DXd works here too. DESTINY-Breast04 showed T-DXd doubled survival compared to chemo in HER2-low patients. Suddenly, nearly half of all breast cancers qualify.
New drugs are coming. Bispecific antibodies like Zanidatamab can hit two HER receptors at once. Early data shows 40% response rates in tough cases. ADCs with even stronger payloads are in phase 3 trials. And researchers are testing T-DXd with immunotherapy - like pembrolizumab - to see if combining immune activation with targeted delivery can push survival even higher.
One trial, DESTINY-Breast06, is testing T-DXd in HER2-ultralow (IHC 0) tumors. If it works, it could expand eligibility to 70% of breast cancer patients. That’s not science fiction. That’s happening now.
How Treatment Is Chosen - And When
There’s no one-size-fits-all. Your treatment depends on stage, location, prior therapy, and health.
Early-stage (before surgery): Trastuzumab + pertuzumab + chemo. Then surgery. Then another year of trastuzumab.
Early-stage (after surgery): If you’re high risk, neratinib for one year. If you’re low risk, just trastuzumab.
Metastatic (spread): First line: trastuzumab + pertuzumab + taxane. Second line: T-DM1. Third line: T-DXd or tucatinib + trastuzumab + capecitabine.
Doctors don’t just pick drugs. They sequence them. Like a puzzle. Each step buys time. Each new drug pushes the deadline further out.
Final Thoughts
HER2-positive breast cancer used to be a death sentence. Now, it’s a chronic condition for many. Targeted therapies have turned a nightmare into a manageable journey. The drugs are smarter. The side effects are more predictable. The survival numbers are better than ever.
But it’s not over. Resistance still happens. Brain mets still creep in. Costs still soar - T-DXd runs about $17,000 a month in the U.S. Access isn’t equal. And new questions keep arising: What’s next after T-DXd? How do we prevent resistance? Can we cure metastatic disease?
The answers are coming. And for the first time, patients aren’t just waiting. They’re part of the research. Their stories, their side effects, their feedback - they’re shaping the next generation of drugs. That’s progress.
What does HER2-positive mean for my prognosis?
HER2-positive used to mean a poor outlook. Today, it means better outcomes than many other subtypes. With modern targeted therapies, survival rates have improved dramatically. Many patients live for years with metastatic disease. The key is starting the right treatment early and sticking with it.
Can I stop HER2-targeted therapy after a year?
For early-stage cancer, yes - trastuzumab is usually given for one year. Stopping earlier increases recurrence risk. For metastatic disease, you usually stay on treatment as long as it’s working and side effects are manageable. Doctors don’t stop unless the cancer progresses or the side effects become too dangerous.
Do all HER2-positive patients get the same treatment?
No. Treatment is personalized. Stage matters. Tumor size matters. Whether it’s spread to lymph nodes or organs matters. Brain involvement? That changes everything. Age, heart health, and prior treatments all play a role. There’s no single protocol - just guidelines. Your oncologist picks the best sequence for you.
Is T-DXd better than T-DM1?
Yes, for most patients who’ve already had trastuzumab and pertuzumab. In the DESTINY-Breast03 trial, T-DXd cut the risk of disease worsening or death by 72% compared to T-DM1. It also doubled median progression-free survival. T-DM1 is still used as a second-line option, but T-DXd has become the new standard after first-line treatment fails.
Can HER2-targeted therapies cure metastatic breast cancer?
Cure is rare in metastatic disease. But long-term control is possible. Some patients live five, seven, even ten years with HER2-positive metastatic breast cancer. Treatments like T-DXd and tucatinib are pushing survival further. While we don’t call it a cure yet, we’re getting closer to making it a chronic, manageable condition.
Rob Turner
I never thought I'd say this, but I'm kinda grateful my sister got HER2+. I know that sounds messed up, but honestly? It meant she got access to all these crazy-smart drugs before most people even heard of them. The fact that we could go from "death sentence" to "manageable condition" in 20 years? That's science doing its job. <3
christian jon
I'm sorry, but I have to say this: If you're still using T-DM1 as second-line? You're doing it wrong. T-DXd is the gold standard now. The data doesn't lie. 72% reduction in progression risk? That's not a statistical fluke-it's a revolution. And if your oncologist is still pushing old protocols? Find a new one. This isn't 2015 anymore.
Autumn Frankart
They say T-DXd works great... but have you seen the FDA warnings? Interstitial lung disease? They're hiding the real cost. Big Pharma doesn't care if you live 2 extra years if they're making $17k/month off you. And don't get me started on how they reclassified HER2-low just to sell more drugs. This isn't medicine-it's a cash grab.
Pat Mun
I'm a nurse who's watched 12 patients go through this journey. Let me tell you something: The emotional toll is just as heavy as the physical. One woman I cared for-61, two kids, metastatic-got on T-DXd. She cried the first time she got the subcutaneous shot because she didn't have to sit in a clinic for 90 minutes anymore. She got to pick up her grandkid from school. That's not just science. That's dignity. And yes, the side effects suck. But the trade-off? Worth it every time.
Skilken Awe
You people act like these drugs are magic bullets. Let's be real: T-DXd has a 10-15% chance of blowing up your lungs. Tucatinib gives you diarrhea so bad you'll rethink your life choices. And don't even get me started on the cardiac toxicity. These aren't treatments-they're high-stakes roulette with a 30% chance of ending up in the ICU. Stop romanticizing chemo.
andres az
I read the paper. The DESTINY-Breast03 trial was funded by Daiichi Sankyo. The median survival increase? Only 18 months. That's not a cure. It's a delay. And they're already pushing it to HER2-low? That's just extending the drug lifecycle. If you're taking this as gospel, you're not thinking critically.
Alyssa Williams
I got diagnosed last year. HER2+. Started trastuzumab + pertuzumab. Had the Phesgo shot. No IV. No 90-minute sit-down. Just a quick jab and I was out the door. My boss didn’t even know I was in chemo. I worked. I took my dog for walks. I ate ice cream. I didn’t lose my hair. I didn’t puke. And yeah, my heart got checked. And yeah, I keep loperamide in my purse. But I’m here. Alive. Working. Living. That’s the win.
Jack Havard
I don't buy any of this. The survival stats are inflated. They're counting people who die 3 months after treatment as "successes" because they didn't die in the first 6. And brain mets? Still a death sentence. They just moved the goalposts. If you're still alive after 5 years, you're a miracle. Not a statistic.
Gloria Ricky
just wanted to say thank you for writing this. i was scared to read it but i needed to. my mom is on t-dxd and i was terrified of the lung thing. but now i know what to watch for. and the part about the subcutaneous shot? i cried. she’s been so tired. this gives us hope. you’re not just a doctor. you’re a light.
Stacie Willhite
My sister’s on tucatinib. Diarrhea’s been brutal. She’s on loperamide 4x a day. But she’s alive. She’s at her daughter’s graduation next month. That’s more than we had last year. I used to think targeted therapy was just fancy chemo. Now I know-it’s not about killing cancer. It’s about letting people live. Even if it’s messy. Even if it’s hard. Even if it costs a fortune. It’s worth it.